CURRENT RESEARCH
BL-Hi-C
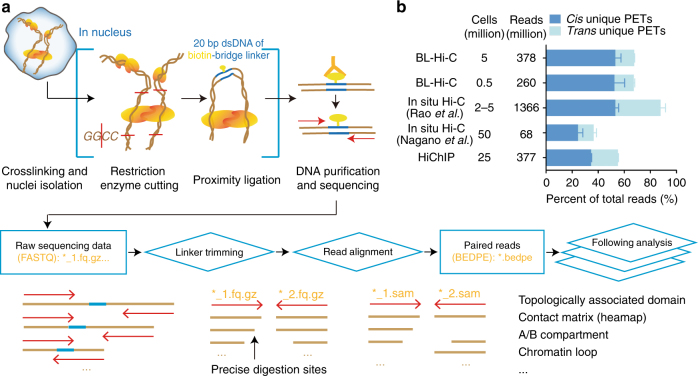
we propose in situ Hi-C method named Bridge Linker-Hi-C (BL-Hi-C) for capturing structural and regulatory chromatin interactions by restriction enzyme targeting and two-step proximity ligation. This method improves the sensitivity and specificity of active chromatin loop detection and can reveal the regulatory enhancer-promoter architecture better than conventional methods at a lower sequencing depth and with a simpler protocol. We demonstrate its utility with two well-studied developmental loci: the beta-globin and HOXC cluster regions.
HiCDB
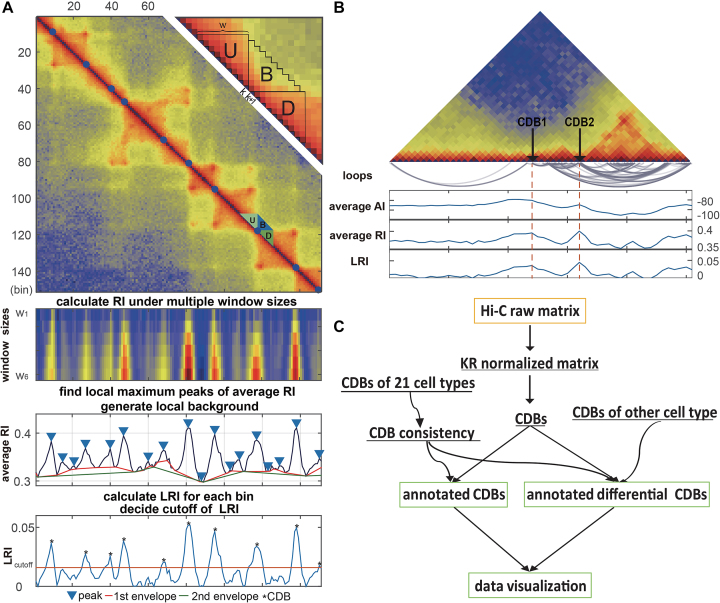
We present a novel method HiCDB, which is constructively based on local relative insulation metric and multi-scale aggregation approach to detect contact domain boundaries (CDBs) on Hi-C map.
This HiCDB method and its related CDB analysis pipeline were implemented as both MATLAB scripts and R package that are available at github link1 and github link2,respectively.
FIND
FIND uses a spatial Poisson process to detect differential chromatin interactions that show a significant difference in their interaction frequency and the interaction frequency of their neighbors. Simulation and biological data analysis show that FIND outperforms the widely used count-based methods and has a better signal-to-noise ratio.
3CPET
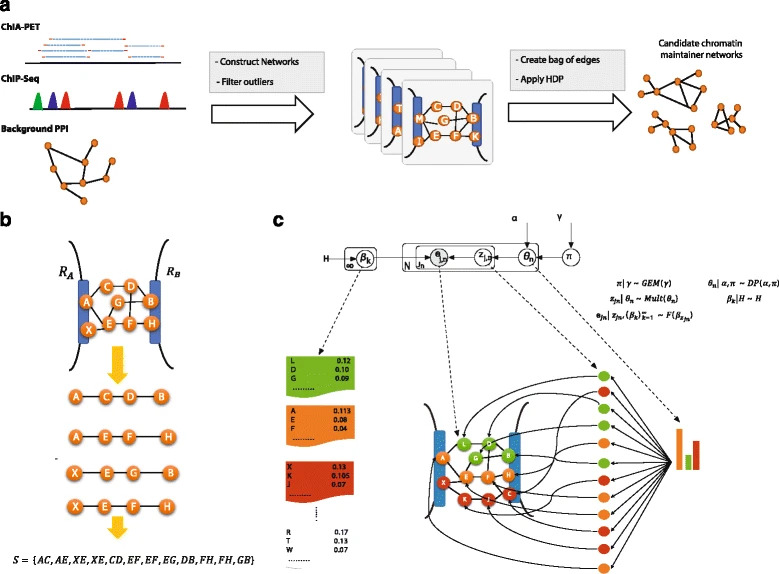
Various efforts have been made to elucidate the cooperating proteins involved in maintaining chromatin interactions; however, many are still unknown. Here, we present 3CPET, a tool based on a non-parametric Bayesian approach, to infer the set of the most probable protein complexes involved in maintaining chromatin interactions and the regions that they may control, making it a valuable downstream analysis tool in chromatin conformation studies. 3CPET does so by combining data from ChIA-PET, transcription factor binding sites, and protein interactions.
SEAM
Spatial metabolomics can reveal intercellular heterogeneity and tissue organization. Here we report on the spatial single nuclear metabolomics (SEAM) method, a flexible platform combining high-spatial-resolution imaging mass spectrometry and a set of computational algorithms that can display multiscale and multicolor tissue tomography together with identification and clustering of single nuclei by their in situ metabolic fingerprints.